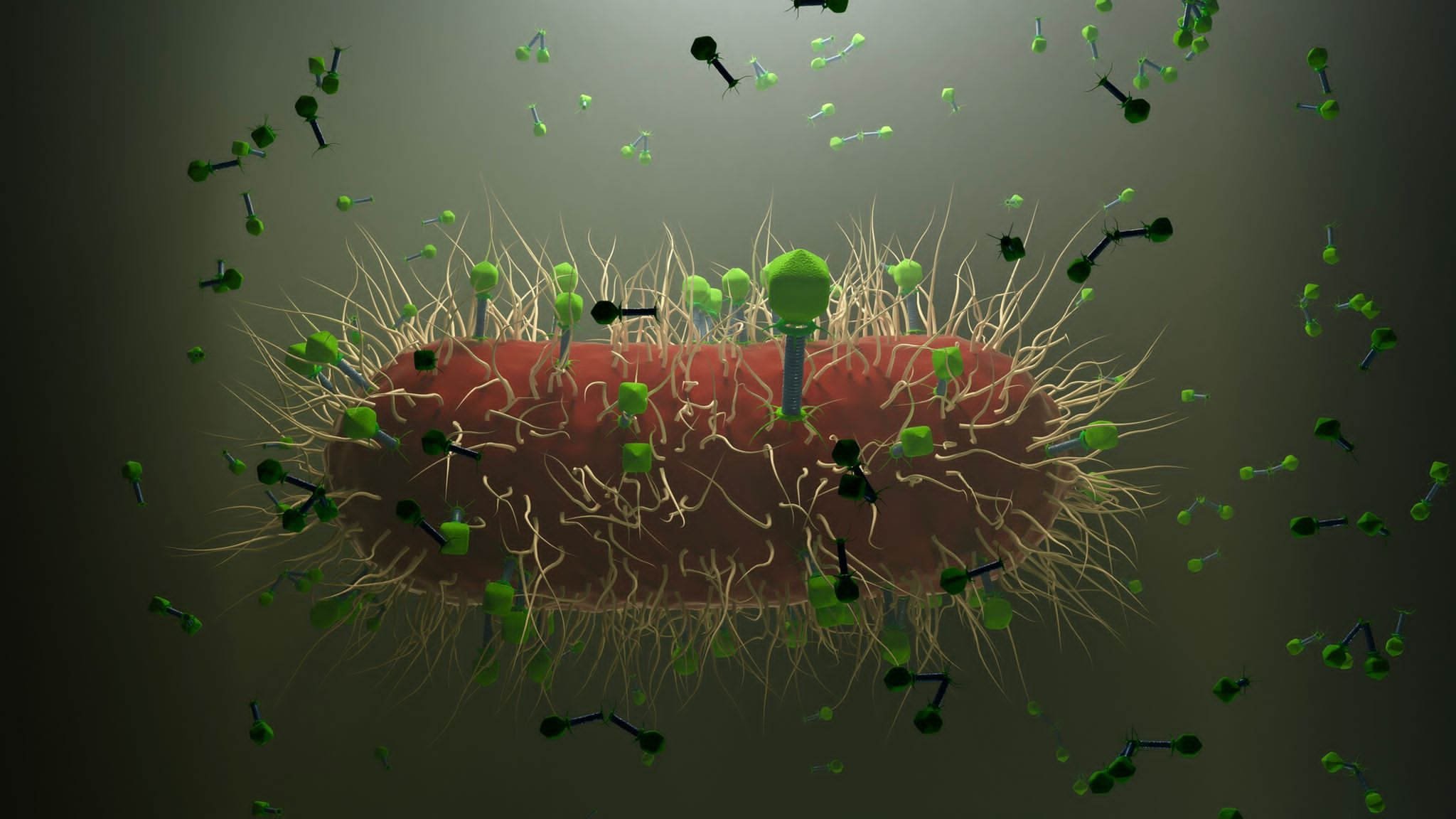
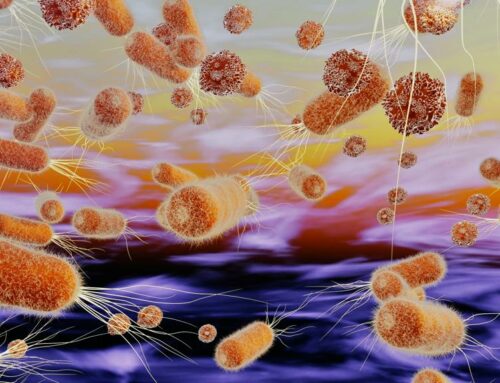
When any bacterium, either beneficial or commensal, harmful or pathogenic, enters the human body system, it is inevitably encountered by molecular defenses of an individual. The intruder provokes recognition mechanisms, activation of our immunity, and potential counter-effects.
Also the host microbiome, a balanced microbial community already existing on epithelia of the host, is getting alerted. The biofilms on human surfaces are structures which have developed into multifunctional organized "microbial states". They have exercised continuous molecular communication with the host and within the "society." Therefore, besides the host defense, an incoming bacterium has to cope with the physico-chemical signals of the microbiota.
On a swarming bacterium, multiple reactivities are in reserve for these interactions. In principle, most bacterial strains strive to balance between their inorganic and organic environments. This objective includes attempts to adapt their metabolism, interactions with microbiomes and production of metabolic compounds, such as organic acids or alcohols, which can stimulate or attenuate other strains. The purpose is to find one's place in the community, not necessarily to overpower the other organisms.
According to some definitions, the microbiome includes, besides the living microbes, viruses, prions, toxins, enzymes, and cell debris of the dead cells. The concept of "microbiota" only designates living and metabolizing cells. The idea of Bacteriological Intestinal Balance (BIB) is ascribed in my book "Alimentary Microbiome – PMEU Approach" (Hakalehto, 2012). Furthermore, there is coming out soon a fascinating piece of scientific literature, the book "I, Microbiome", written by several experts in the field (and Vladimir Jakovljevic as a lead author).
The balance of microbial communities is also beneficial for the human host. The former usually change their growth modes between the attached or biofilm-integrated cells and the swarmer cells (Hakalehto, 2015). The well-functioning and elaborated molecular communication between various bacteria, microbes, and man are increasing the microbiome's diversity. At the same time, microbiomes become increasingly integrated with human tissues, organs and their regulation. Therefore, it is most natural for the body system to influence the microbiome through various molecular signals and adjustments.
Coming to the role of viruses in biological systems, they are also inherent and specific to bacteria and other microbes. They represent a form of partially diverged self-control, also transferring chemical messages and genetic codes. As viruses are found in practically all species of plants, animals and microbes, it is justified to suppose that they indeed fulfil some omnipotent roles in nature. Could it be, as many suspect, that they serve as vehicles for regulating populations? This could be doubted, and then the population would be deliberately self-destructive, which is not a common feature among known living entities. In other words, a mother lion is sacrificing herself to protect her cubs, or a bison bull may get lethal wounds in a fight with another, but they have a gain to be achieved. That could be called purpose.
Viruses, such as bacterial viruses, the bacteriophages, deliver messages related to ecological adaptation, metabolic performance, spatial distribution etc. What then turns viruses into disease-causing agents in human, animal or plant cells? Moreover, they are derived from infected cells of the same kind. So, we may well ask if the purpose is self-restriction on the host species level if the growth limits have been met? Then, the specific community of metabolizing cells could get equipped against destruction. However, this seems not always be the case, as the current pandemic is spreading, although the population should mainly defend itself by natural or acquired defenses.
We could deduce that biological purpose of viruses is not fully understood yet, still we could use them as biotechnological tools. We could learn from bacterial viruses. Bacteriophages could replace antibiotics, be bioindicators, or transfer medicines and genetic material (Rogovski et al., 2021). They belong to the laboratories of biotechnological research, but they have shaped our biological world to a great extend and continue to do so.
Author:
Elias Hakalehto, Adj.Prof., PhD
CEO, Finnoflag Oy
Alumnus, University College London (biochemical engineering)
Vice President, International Society of Environmental Indicators
Fellow member (lifetime), International Society for Development and Sustainability (ISDS) (Japan)
Mobile phone +358-500-574 289
Edited by Dr. Vladimir Jakovljevic
CEO, Microbiome Power
Literature: